Home
RaphaŽl Robiette
Research
People
Publications
Teaching
Contact Details
Links
Intranet

The Robiette Research Group
Research in Organic and Physical Organic Chemistry
Medicinal Chemistry
Synthesis and biological evaluation of novel imidazole-containing macrocycles
In the context of a collaboration with industry, we have designed and synthesised novel 5-aryl-1H-imidazoles contained in a macrocycle (see Tetrahedron 2010, 4515). The synthesis of these original compounds has necessitated the development a divergent and regioselective strategy toward 1,2,4- and 1,2,5-trisubstituted imidazole derivatives (see methodology pages). The biological evaluation of these macrocycles revealed that they display actual binding activity towards four important biological targets: A3 adenosine (h) receptor, dopamine D1 (h) receptor, chloride channel (GABA-gated) and choline transporter (h) CHT1.
We also studied the chiral properties of these macrocycles (atropisomerism) using experimental (Chiral HPLC and NMR) and computational (DFT) methods (see physical organic pages).
Development of new anticancer agents
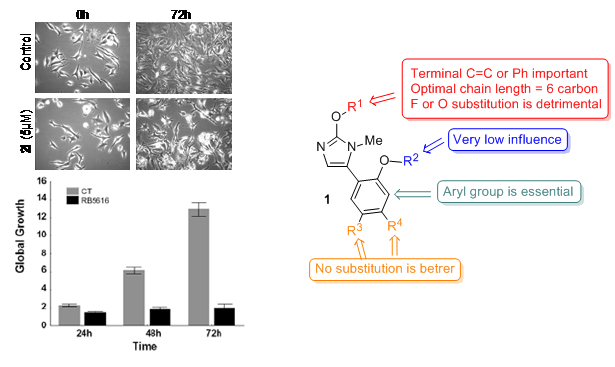
Several cancers are still associated with bad prognosis because of their resistance to pro-apoptotic stimuli (and thus to most chemotherapeutic insults). In this context, in collaboration with Prof. V. Mathieu and Dr R. Kiss (ULB, Brussels), we discovered that 5-aryl-1H-imidazoles such as 1 (which are related to intermediates in the syntheis of our macrocycles, see above) are potential anticancer agents (see J. Med. Chem. 2013, 6626). Indeed, determination of the in vitro growth inhibitory activity of these compounds in various cancer cell lines revealed that they display cytostatic anticancer activity with IC50 in the order of single digit micromolar. Interestingly, the most active compounds were as active on apoptosis-resistant cancer models than on apoptosis-sensitive ones. The large panel of compounds synthesized and tested allowed us to draw some conclusions concerning the structure-activity relationship.
Unexpectedly, thanks to a screening by National Cancer Institute (NCI) we discovered that the profile of our lead compound is not correlated to any anti-cancer compound in the NCI database, meaning that its mechanism of action should be different of the one of all cell lines in this database. The use of transcriptomic characterized melanomia models enabled Prof. V. Mathieu to highlight mitochondrial targeting by our compounds. This hypothesis was further confirmed by reactive oxygen production measurement and oxygen consumption analysis.
Design, synthesis and pharmacological evaluation of novel selective inhibitors of hFAAH
To be added.
This page and all its contents belong to and were written by RaphaŽl Robiette.
For any comments or suggestions, please contact me.