The Robiette Research Group
Research in Organic and Physical Organic Chemistry
Methodology
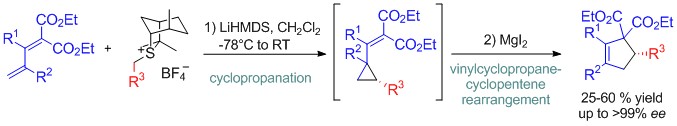
Development of an asymmetric (4+1) annulation reaction between sulfur ylide and 1,3-diene
Drawing on our experience in both cycloaddition reactions and onium ylide chemistry, we developed an asymmetric (4+1) annulation strategy allowing the highly enantioselective synthesis of functionalized cyclopentanoids . Our methodology consists in a cyclopropanation reaction between sulfur ylides and 1,3-dienes followed by a, in situ, rearrangement of vinylcyclopropane into desired cyclopentenes (see Chem. Eur. J. 2015, in press).
In this process, stereoselectivity of the cyclopropanation is controlled by the chirality of the sulfur ylide. Interestingly, the subsequent MgI2-catalyzed vinylcyclopropane rearrengemnt takes place with a total stereospecificity. This stereospecific character could be explained by iodode-assisted ring opening through a SN2 mechanism followed by intramolecular SN2 alkylation, leading thus to an overall retention of configuration for the chiral centre.
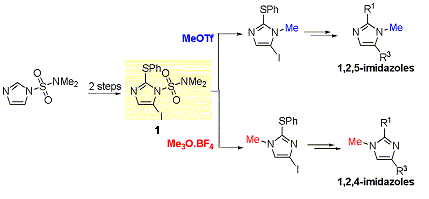
Development of a general and highly regioselective N-methylation of azoles
In the context of the synthesis of biological active compounds (see medicinal chemistry page), we have been interested in the synthesis of trisubstituted imidazole derivatives. We have developed a divergent and regioselective synthesis of 1,2,4- and 1,2,5-polyfunctionalized imidazole derivatives from a readily available (2 steps) common intermediate (1). Our startegy is based on a regioselective N-methylation of this latter (see J. Org. Chem. 2008, 6816).
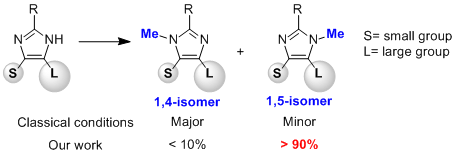
We then have further developed our methodology to obtain a highly regioselective N-methylation process of (NH)-(benz)imidazoles furnishing the more sterically hindered and usually minor regioisomer (see J. Org. Chem. 2014, accepted).
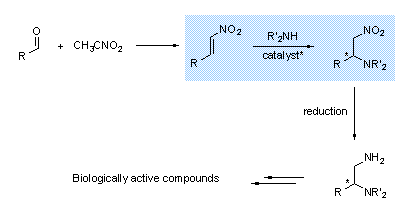
Synthesis of 1,2-diamines
We develop also a stereoselective strategy for obtaining beta-nitroamines via MichaŽl addition of a N-nucleophile on nitroalkenes.
This page and all its
contents belong to and were written by RaphaŽl Robiette.
For any comments or suggestions, please contact me.